Fat in the human body is located in subcutaneous and the visceral compartment. While subcutaneous fat is stored predominantly in the abdominal wall and the lower body segment (femoral and gluteal area), the visceral fat is located in the peritoneal (greater omentum, lesser omentum and mesenteric fat) and retroperitoneal locations. Visceral adipose tissue accounts for 20% of the total body fat in men and ~8% in women. Am J Clin Nutr. 1994 Jun;59(6):1277-85. The upper body fat encompasses the visceral fat and the subcutaneous fat of the abdominal wall.
|
Visceral adipose tissue (VAT) is associated with the individual components of the metabolic syndrome in contrast to subcutaneous adipose tissue. The association of the visceral adipose tissue with obesity-related complications has been recognised as early as 1956. Am J Clin Nutr. 1956 Jan-Feb;4(1):20-34 ; Since then, insulin resistance, J Clin Invest. 1984 Oct;74(4):1515-25 diabetes Diabetes. 1985 Oct;34(10):1055-8. hypertension J Chronic Dis. 1987;40(5):421-8. dyslipidaemia J Clin Epidemiol. 1991;44(2):183-90. and cardiovascular morbidity and mortality Br Med J (Clin Res Ed). 1984 Nov 10;289(6454):1257-61. have all been shown to be related to the extent of visceral adipose tissue. While the products secreted by adipocytes or macrophages in the visceral and subcutaneous adipose tissue is discussed in detail elsewhere at this site and accessible through the links at the top of this page, the non-endocrine contribution of the visceral adipose tissue to the pathogenesis of insulin resistance and the resultant unfavourable hyperglycaemic and hyper-triglyceridaemic milieu is discussed here.
|
Removing the visceral fat of obese rats improves hepatic insulin sensitivity with resultant reduction in hepatic glucose output. Diabetes. 1999 Jan;48(1):94-8. While this emphasises the unfriendly nature of the visceral adipose tissue, it does not clarify the pathogenetic mechanisms that initiated the disturbances in fat distribution or glucose metabolism. Much debate still goes on about the pathogenesis of the insulin resistant state. Perhaps a few thoughts are in order before proceeding to look at the various hypothesis available. In clinical practice, physicians are still amazed by morbidly obese patients who surprisingly remain euglycaemic or in other words, "fat yet fit". On the other hand, patients with lesser degrees and duration of obesity become diabetic. What is the explanation for this paradox? Are there protective factors against insulin resistance in those lucky obese? Or is it that deleterious factors are present in those unlucky diabetic obese? Or is there a threshold level of fat that each individual is capable of handling before slipping inexorably into dysglycaemia such that all obese humans are likely to become diabetic once they reach their tolerable degree of fat? And then there is the 5% of type 2 diabetics who are not obviously obese clinically. To try to explain these unexplainables, newer terms and hypothesis have been offered which will be the subject of the rest of the page. These centre around the distribution of the fat and the resultant changes brought about in insulin action at the level of the muscle, adipose tissue and β cells.
|
Taking one question at a time, are there protective factors against insulin resistance in those lucky non-diabetic obese? Subcutaneous fat is now recognised to secrete adiponectin which is an insulin sensitising agent with anti-inflammatory properties, with lower secretion of the same from visceral adipose tissue. Adiponectin facilitates fatty acid oxidation in peripheral tissues and could protect against fat deposition outside of the adipose tissue thus preventing development of insulin resistance. (see ectopic fat storage syndrome below) Type 2 diabetics have lower levels of adiponectin levels correlating with visceral obesity. Leptin, another hormone secreted predominantly from subcutaneous adipose tissue as opposed to visceral adipose tissue, Diabetes. 1998 Jan;47(1):98-103 is thought to be involved in appetite modulation as part of its short term meal regulation function as well as improving insulin sensitivity. Leptin secretion increases with increasing adipocyte cell size. Hyperleptinaemia facilitates suppression of appetite through central mechanisms while facilitating fat oxidation in skeletal muscles and preventing lipogenesis by down regulating SREBP-1c. Endocr Rev. 2002 Apr;23(2):201-29. thus preventing fat deposition in non-adipose tissues. Testosterone levels in men seems to inhibit fatty acid uptake in visceral adipose tissue, Metabolism. 1995 Sep;44(9 Suppl 3):21-3 a beneficial effect that could be lost with advancing age in this sex. The high density of androgen receptors in visceral adipose tissue in men Am J Physiol. 1998 Jun;274(6 Pt 1):C1645-52. is in keeping with this hypothesis. In women, the estrogens promote subcutaneous fat accumulation (gluteo-femoral) with increased adiponectin production. Post menopausally, the loss of estrogen results in increased visceral obesity Ann Intern Med. 1995 Nov 1;123(9):673-5. with lower subcutaneous adipose tissue, often with unchanged weight profiles.
|
Are there deleterious factors present in those unlucky diabetic obese? Macrophages in adipose tissue increases with increasing adipose tissue size. J Clin Invest. 2003 Dec;112(12):1821-30. Visceral fat is known to produce a variety of inflammatory substances thanks to the macrophages present in the tissue namely TNF alpha, TGF β, Interleukin 6, as well as resistin, plasminogen inhibitor 1, and angiotensinogen; all factors that decrease insulin sensitivity. On an endocrine and paracrine basis, these could facilitate an unfavourable metabolic milieu. Direct drainage of the visceral fat products and free fatty acids into the portal circulation Arteriosclerosis. 1990 Jul-Aug;10(4):493-6. has been thought to result in development of non-alcoholic steatohepatitis and hepatic insulin resistance, although tracer studies seem to show that NEFA concentrations are almost similar in portal and systemic circulation, J Clin Invest. 1970 Nov;49(11):2017-35 with only 10% of the NEFA delivered to the liver being from the visceral fat. Post prandially, splanchnic blood flow increases with rise in catecholamine levels which could increase NEFA delivery into the portal circulation. Visceral fat also demonstrates higher number of beta1 and beta 2 adrenergic receptors J Clin Endocrinol Metab. 1992 Jul;75(1):15-20. with higher rates of catecholamine mediated lipolysis, which is less effectively inhibited by insulin at this site, a characteristic probably explained by reduced insulin substrate protein (IRS) expression in visceral adipose tissue. Diabetologia. 1998 Nov;41(11):1343-54. At the same time, despite a resistance to antilipolytic action of insulin, the visceral adipose tissue probably remains sensitive to insulin-induced glucose uptake and utilisation through increased GLUT-4 expression, J Clin Endocrinol Metab. 2004 Jun;89(6):2989-97. thus favouring continued unutilised triglyceride accumulation in the visceral adipose tissue. The increased 11 β HSD activity in visceral adipose tissue Metabolism. 1995 Sep;44(9 Suppl 3):21-3 along with higher glucocorticoid receptor density in VAT J Clin Endocrinol Metab. 1990 Nov;71(5):1215-9. increases local cortisol levels with further increase in triglyceride storage through lipoprotein lipase modulation. J Clin Invest. 1993 Nov;92(5):2191-8. Accumulation of visceral adipose tissue also seems to increase the fat storage in subcutaneous tissue J Clin Endocrinol Metab. 2004 Mar;89(3):1379-84. and at a later stage of the disease increases lipolysis rate in subcutaneous adipose tissue of the abdominal wall by making it resistant to insulin's anti-lipolytic action Am J Physiol Endocrinol Metab. 2001 Jan;280(1):E40-9 while preserving insulin sensitivity for glucose uptake, forcing it to behave functionally as visceral adipose tissue. Induction of PPARγ2 in subcutaneous adipose tissue by RXRα , αSREBP1 and SREBPc is thought to contribute to the increased fat storing properties of subcutaneous adipose tissue. VAT has also been shown to have a lower rate of apoptosis in its adipocytes compared to subcutaneous adipocytes Diabetes. 1998 Aug;47(8):1365-8. which might be related to the expression of the "cellular inhibitor of apoptosis-2", a protein that inhibits TNF alpha mediated cell death, Diabetes. 1998 Sep;47(9):1384-91. thus increasing the number of unfriendly VAT adipocytes.
|
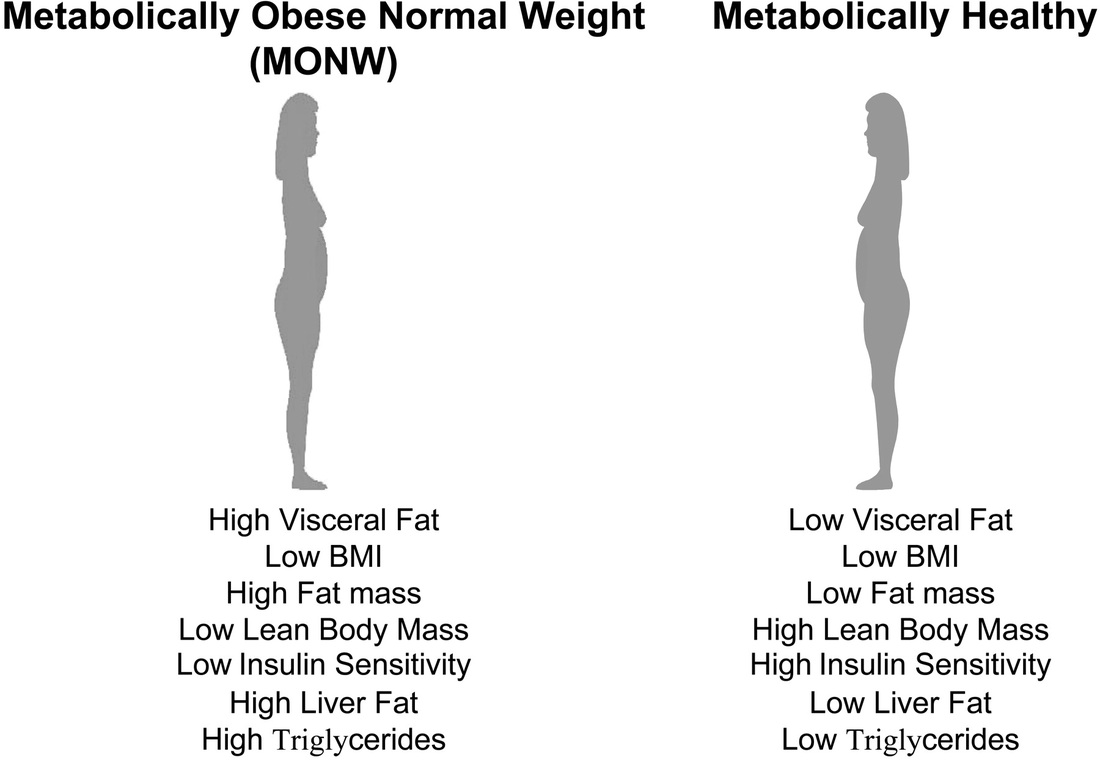
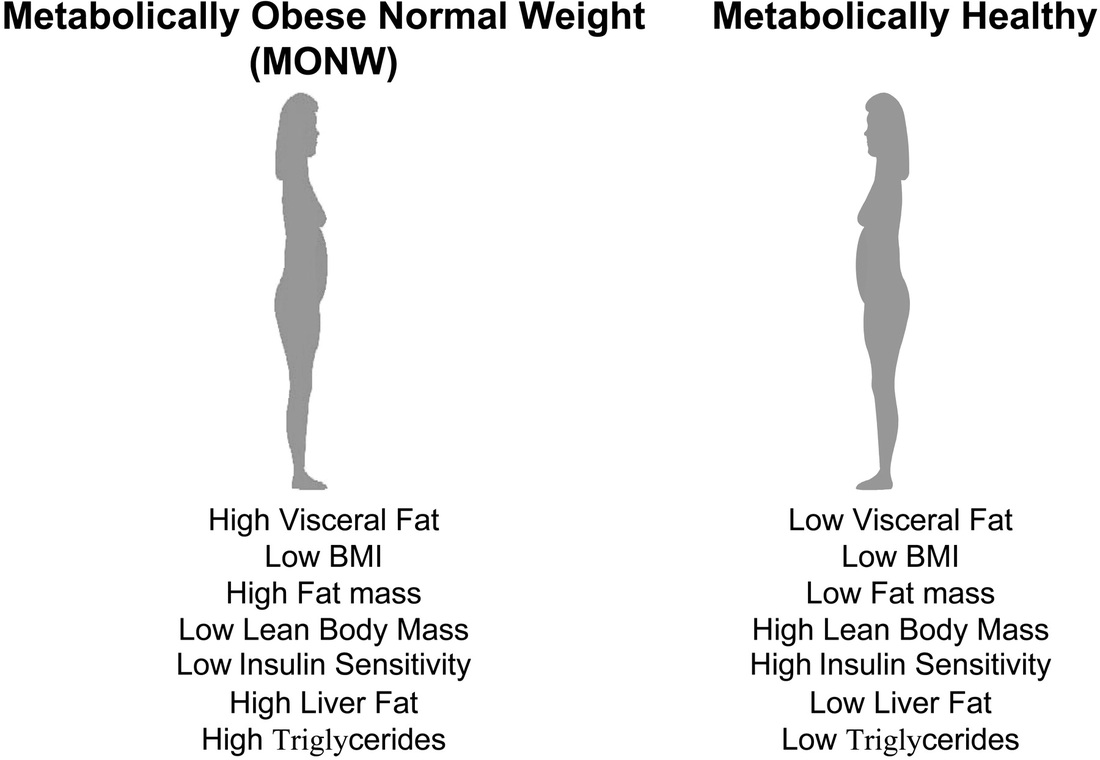
Is there a threshold level of fat that each individual is capable of handling before slipping inexorably into dysglycaemia? Thoughts in this vein has stimulated the coining of new terms namely, Metabolically Normal Obese (MNO) or Metabolically Healthy Obese (MHO) and the Metabolically Obese Normal Weight (MONW). Diabetes. 1998 May;47(5):699-713. It is now postulated that there is probably a Critical Visceral Adipose Tissue Threshold (CVATT) that a person has to reach before insulin resistance results. Attainment of this CVATT probably diverts fat deposition from visceral adipose tissue to muscle-intramyocellular lipid deposition- and the liver with resultant insulin resistance in these organs. Diabetes. 2002 Jan;51(1):7-18 What determines this CVATT level if it exists is not clear yet. But it certainly would help explain the lean type 2 diabetic (MONW). VAT accumulation is associated with metabolic disturbances even in clinically non-obese individuals. Am J Clin Nutr. 1981 Aug;34(8):1617-21. Based on imaging studies it has been suggested that a visceral adipose tissue level >110cm2 would lead to risk of coronary heart disease Int J Obes Relat Metab Disord. 1996 Jul;20(7):613-7. while >130cm2 would predispose to metabolic disturbances. Diabetes Care. 2003 May;26(5):1413-20. It is possible that these MONW individuals attained insulin resistance early, limiting further fat deposition in adipose tissue with earlier occurrence of the ectopic fat storage syndrome. What predisposes some individuals to a predominant accumulation of VAT without significant subcutaneous fat accumulation is unknown presently although ethnic origin (Japanese or Asian) Obes Res. 2001 Jul;9(7):381-7. as well as low birth weight and low weight at one year of life have been suggested as predictive factors. Int J Obes Relat Metab Disord. 2002 Jan;26(1):40-7. In this context, it is probably thought provoking to note that induction of muscle-specific insulin resistance in mice results in development of increased central adiposity. Endocrinology. 1996 Jun;137(6):2397-405. Thus does peripheral insulin resistance contribute to central obesity?
|
On the other side of the spectrum are patients who may have higher amounts of subcutaneous fat as opposed to visceral fat. (MNO) . One could not look at a Japanese Sumo wrestler and not be amazed to realise that he is not diabetic despite consuming 5000 calories a day to maintain his obesity! The paucity of visceral fat probably explains this phenomenon. Diabetes Metab Rev. 1997 Mar;13(1):3-13 It should be noted that the presence of abdominal obesity while being the best clinical marker of insulin resistance need not necessarily reflect proportionally high visceral fat in all individuals, as subcutaneous fat might be predominantly higher in a particular individual, making him/her less predisposed to development of the diabetic state. Increased fat deposition in the subcutaneous tissues in these subjects might help them avoid reaching or exceeding their CVATT, thus preventing development of metabolic disturbances despite being obese. Exercise could be crucial in ensuring that the fat remains in the subcutaneous depot, well demonstrated by the fact that sumo wrestlers tend to develop insulin resistance in retirement! Ethnicity again may play a role in determining whether the accumulated visceral adiposity produces deleterious effects including cardiovascular risk. Am J Epidemiol. 1996 Mar 1;143(5):442-55. Adipocyte size may also influence development of metabolic disturbances as older more mature fat-replete adipocytes are more likely to be insulin resistant as opposed to newer younger smaller adipocytes. Mol Genet Metab. 2001 Mar;72(3):231-8. It has been proposed that this group of metabolically normal obese might constitute up to 20% of the obese population, J Clin Endocrinol Metab. 2004 Jun;89(6):2569-75 although regional variations have yet to be established.
|
So how does reaching CVATT result in development of insulin resistance? Apart from insulation, adipose tissue is thought to be intended by nature as a storage organ for fat to serve as a reservoir of energy in times of starvation. Deposition of fat in tissues other than adipocytes would have been unintended by nature which did not anticipate the rate of progress that man would achieve with the founding of McDonalds! Fat storage in adipocytes is a function of size rather than number. In other words, fat storage in adipose tissue is managed by increasing storage within existing adipocytes rather than increased rate of production of new adipocytes. Pre-adipocytes which are differentiated into adipocytes enthusiastically store fat and transform into mature adipocytes which reach their limit for fat storage and become insulin resistant. When preadipocytes are no longer available for further mopping up of the circulating fatty acids, the storage capacity of adipose tissue is exceeded and the fatty acids are diverted to muscles and liver which serve as unwilling buffers recruited to cope with the progressive exogenous (dietary) or endogenous (hepatic production) lipid influx. Triglyceride deposition in these organs evokes insulin resistance at the muscle and hepatic level and is referred to as the "ectopic fat storage syndrome". When these "inexperienced" organs are overwhelmed, free fatty acid level in circulation increase, with further worsening of insulin resistance. This mechanism might well apply to those humans with lipodystrophic syndromes who lack subcutaneous fat and hence have excess triglyceride deposition in the muscle and liver with insulin resistance development. Trends Endocrinol Metab. 2000 Dec;11(10):410-6. Similar mechanisms clearly operate in the lipoatrophic mouse, well demonstrated by improvement of metabolic parameters on surgical implantation of adipose tissue. J Clin Invest. 2000 Feb;105(3):271-8. Intramyocellular lipid content has been shown to be higher in type 2 diabetic patients compared to equally obese non-diabetic subjects Diabetologia. 2001 Jul;44(7):824-33. and in lean subjects, Diabetes. 2001 Apr;50(4):817-23. with reduced capacity for fat uptake and oxidation of fatty acids during fasting and exercise. Am J Physiol Endocrinol Metab. 2000 Jul;279(1):E146-54. Thus despite the hypothesis of fat overflow into non-adipose tissues, it remains a possibility that the intramyocellular triglyceride accumulation could be a primary problem due to defective fat oxidation in muscle. Inhibition of CPT-1 (Carnitine palmitoyl Transferase-1) in rats results in decreased fat oxidation in muscle with intramyocellular lipid accumulation and resultant insulin resistance. Diabetes. 2001 Jan;50(1):123-30. It is possible that the chronic exposure to free fatty acids in circulation elevates malonyl CoA levels leading to CPT-1 inhibition with decreased fat oxidation in muscle, Am J Physiol Endocrinol Metab. 2000 Aug;279(2):E259-65. bringing us back to the original theory of fat overflow.
|
So is it possible to increase the number of pre-adipocytes transforming into adipocytes, such that the ability to keep fat inside adipose tissue is increased? Subcutaneous adipose tissue has a higher capability for pre adipocyte differentiation than visceral adipose tissue. Preadipocyte differentiation is at least partly regulated by PPAR (peroxisome proliferator activated receptors). J Clin Invest. 1997 Dec 15;100(12):3149-53. Stimulation of PPAR by thiazolidinediones can thus facilitate preadipocyte differentiation, in turn increasing the number of insulin sensitive adipocytes capable of further fat storage in the adipose tissue. This decreases the circulating free fatty acids and the deposition of fat in non-adipose tissue organs. The stimulation of preadipocyte differentiation mediated by thiazolidinediones is preferentially in the subcutaneous fat, J Clin Invest. 1997 Dec 15;100(12):3149-53. facilitating redistribution of fat from the dangerous visceral area to the subcutaneous area. Diabetes Res Clin Pract. 2001 Dec;54(3):181-90. In fact, this increased subcutaneous fat deposition is partly responsible for the weight gain induced by thiazolidinediones. Interestingly the effectiveness of thiazolidinediones in preadipocyte differentiation in subcutaneous tissue cannot be attributed to PPAR gamma receptor density as both subcutaneous and visceral adipose tissue seems to have similar density of these receptors. Diabetes.1998 Sep;47(9):1384-91. Patients with a form of lipodystrophy with excess fat storage in subcutaneous tissue- multiple symmetric lipomatosis- remain insulin sensitive despite increased fat accumulation emphasising the metabolically friendly nature of the subcutaneous adipose tissue. Diabetes Care. 2004 Mar;27(3):794-5.
|
Contradictory to the hypothesis of visceral fat overload influencing hepatic fat deposition is the demonstration that weight loss of similar amounts in different groups with variable dietary fat resulted in differing degrees of liver fat reduction which was not related to visceral fat loss. Diabetes. 2003 Mar;52(3):701-7. Thus dietary fat is suggested to be a more important determinant of liver fat than the amount of visceral fat itself.